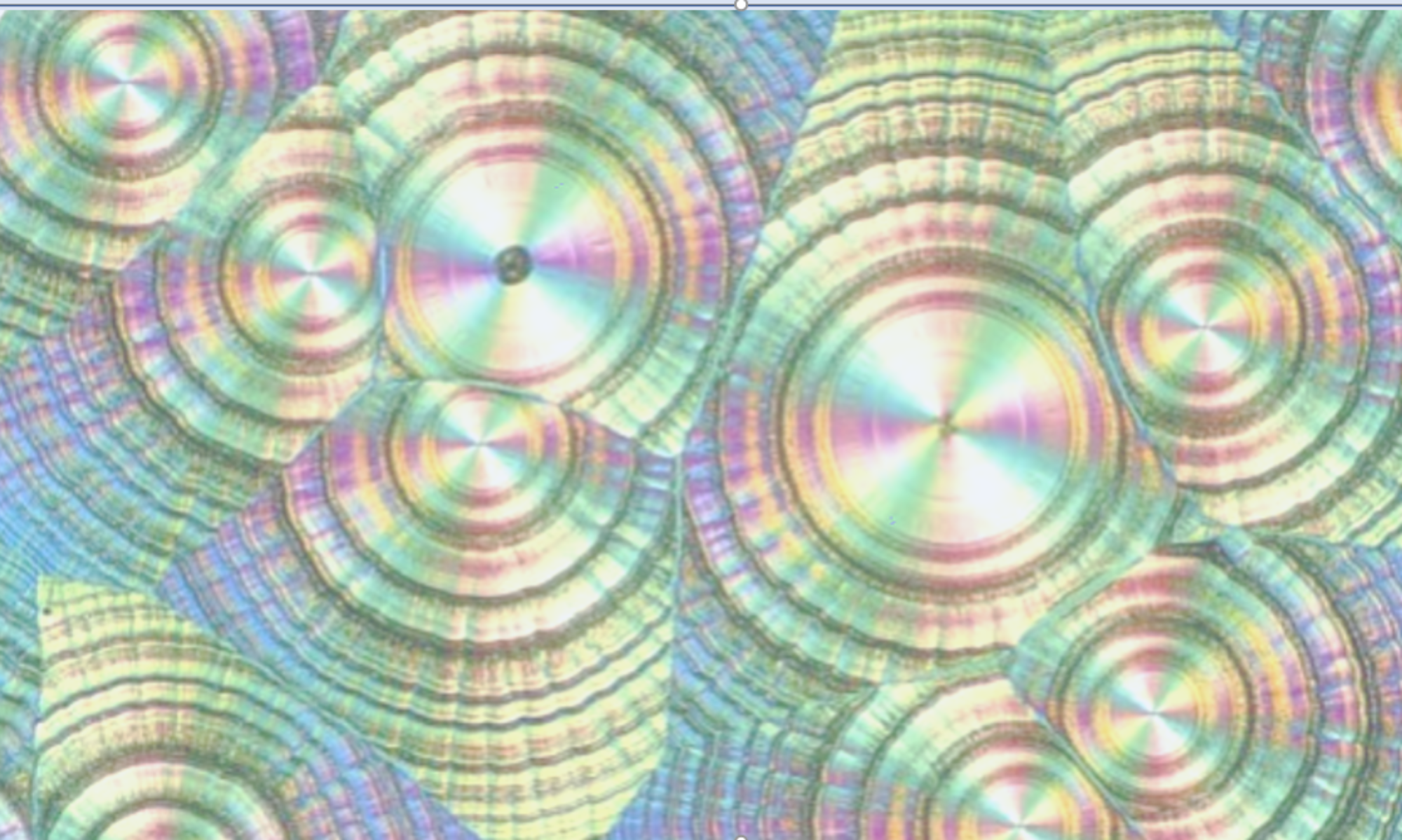
Selecting the right version for clinical studies is the single most important solid state decision. Changing version during development can lead to costly bioequivalence studies. Many CROs operate in this space and it is important to ensure the right work is done and that data is interpreted correctly.
Example of Skills and Experience
I led a team accountable for around 30 version screens per year. This gave me a portfolio view of version selection to optimise Biopharmaceutics, Stability and Manufacturability. As an example, a BCS class 1 oral solid dosage form exhibited poor chemical stability due to the initial selection of a version with too high aqueous solubility. This may have meant the molecule was undevelopable. To solve the problem, the API was rescreened and a less soluble version identified and fully characterised. Stability studies indicated that this was more stable than the initial version. Switching to this less soluble version was crucial in keeping the project on track.
•For a similar example see https://doi.org/10.1002/jps.22009