QbD is the industry standard expected in any regulatory filing for commercial approval and describes the underlying science and process controls required for the API.
Example of Skills and Experience
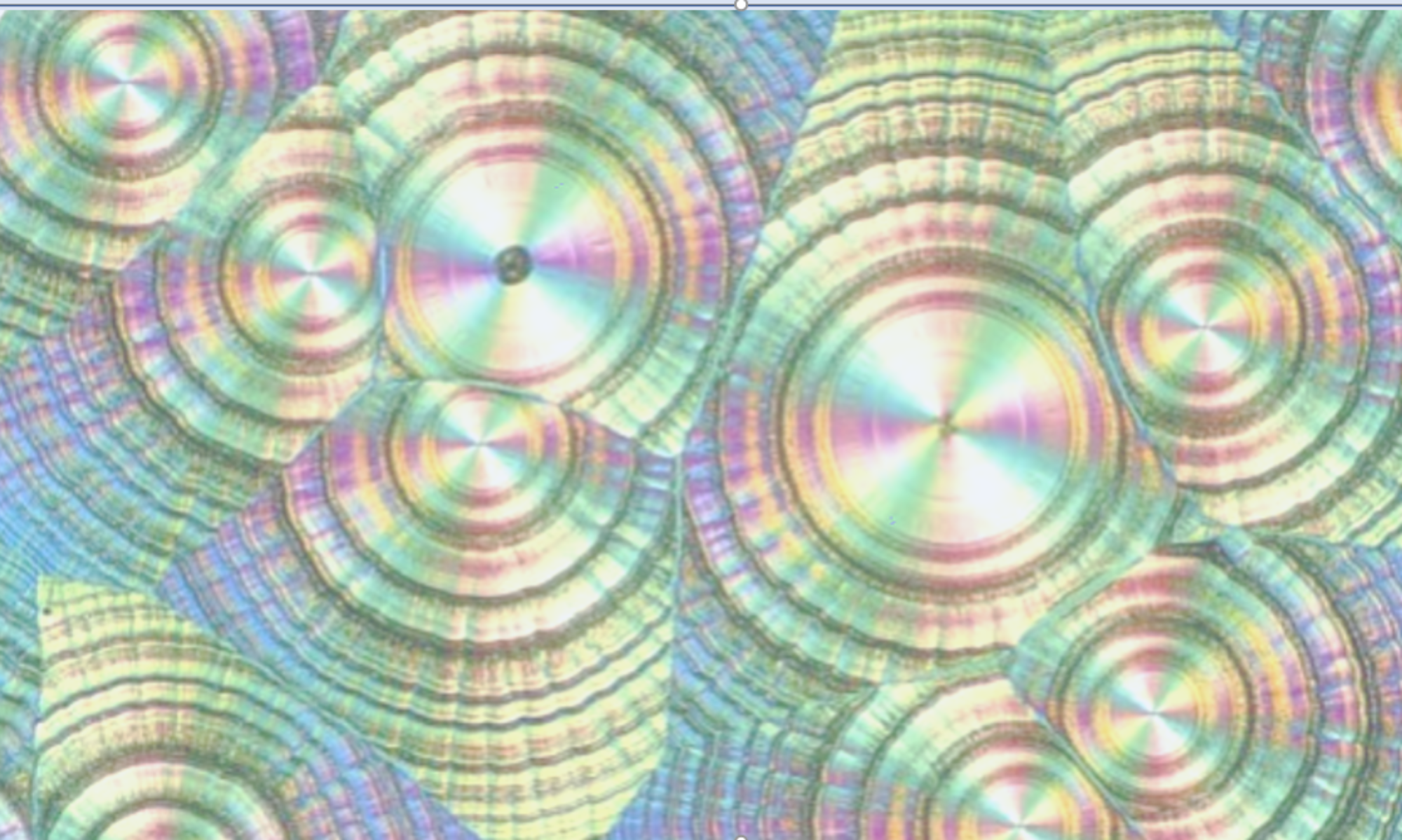
A QbD package was required for a novel anti-malarial which described the critical quality attributes and the associated process parameters for the process. I directed the experimental strategy and developed the relevant sections of the regulatory filing. This included a full understanding of the solid state landscape, justification for API colour and the control of API PSD through a seeded cooling crystallisation using micronized seed.
See https://doi.org/10.1021/op100209c for an example of QbD in crystallisation.