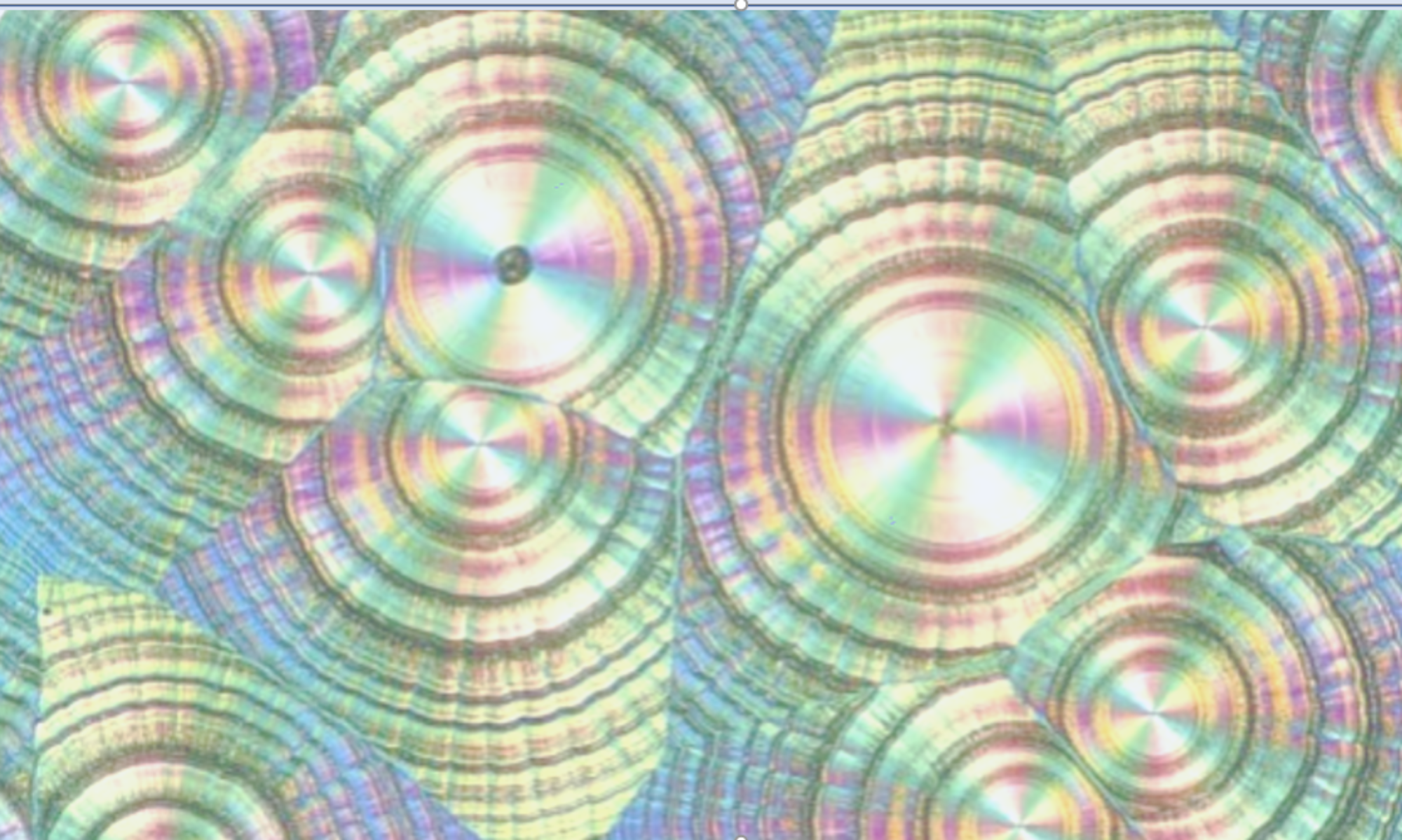
Like polymorphism, hydration and solvation can impact bioavailability and potentially manufacturability. The occurrence should be well understood and if needed, control imparted in the API step, the formulation or on storage so the drug reaches the patient in a consistent hydration state.
Example of Skills and Experience
An oral antibiotic in phase 2 and on an accelerated development plan exhibited a series of failures on tablet disintegration testing. These failures were associated with a change in chemical route which resulted in only minor differences in the API impurity profile. I led the investigation which discovered that a hitherto unknown hydrate was formed on contact with water. The hydrate had an extreme needle habit which prevented the correct functioning of the superdisintegrant and this in turn led to disintegration failure. Both the API crystallisation and the formulation were redesigned to circumvent this. As a result, pivotal studies started as planned.
See https://doi.org/10.1002/jps.24516 for a similar example for Clarithromycin.